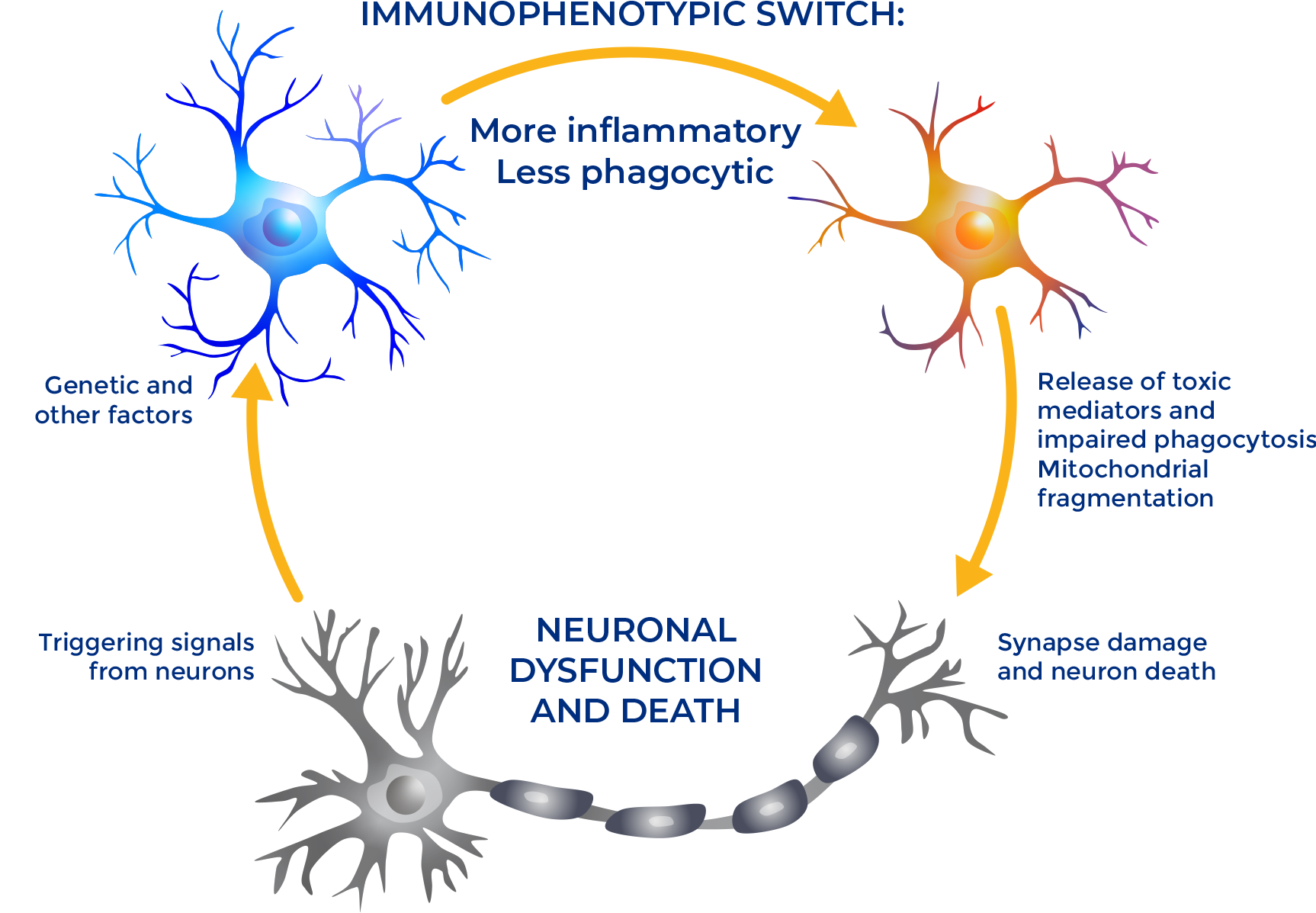
Myeloid immune cells play an early and critical role in the pathophysiology of multiple immune-mediated and mitochondrial neurological diseases. Their normal metabolic function can become impaired and overwhelmed by damaging foreign or toxic substances, often triggered by aberrant signaling from key regulators like PGC1α. Once these cells become inflammatory, they contribute to the degeneration of neurons—leading to a host of potential diseases.
Because our platform targets fundamental cellular processes present in all mammalian cells, it appears to have clinical utility across a broad range of immune-mediated and mitochondrial diseases—beyond the CNS.
We believe we have discovered a potentially disease-modifying approach to the treatment of both CNS-related and systemic diseases—one that can help halt and potentially reverse disease progression and enable patients to live better lives.